Middle Eastern and North African Active Pharmaceutical Ingredient (API) Market Trends and Forecast
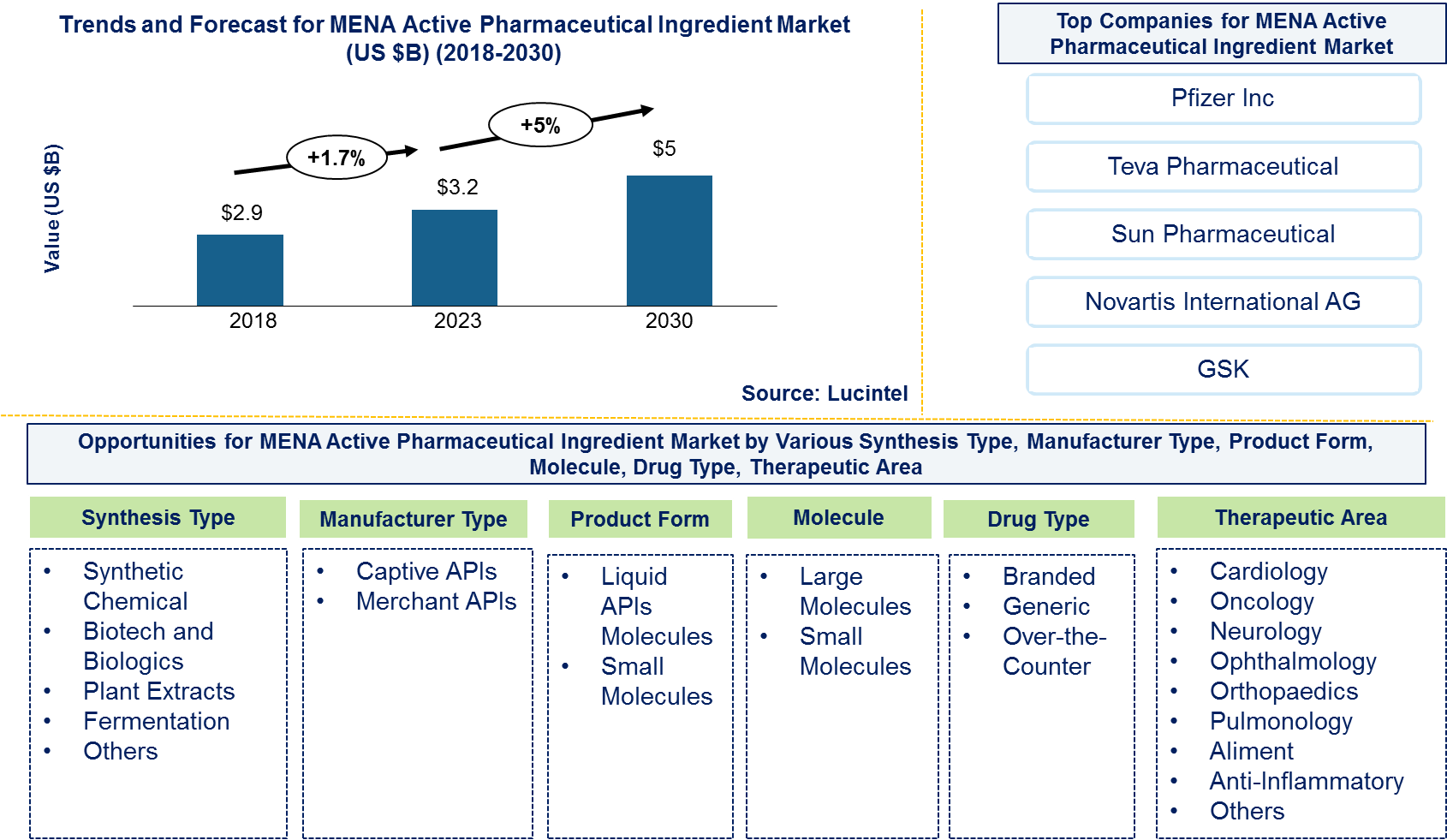
The future of the Middle Eastern and North African active pharmaceutical ingredient market looks promising with opportunities in cardiology, oncology, neurology, ophthalmology, orthopedics, pulmonology, aliment, anti-inflammatory therapeutic areas. The global MENA API market is expected to reach an estimated $5 billion by 2030, and it is forecast to grow at a CAGR of 5% from 2023 to 2030. The major drivers for this market are rise in growth of pharmaceutical production and research and development of new drugs.
Raw materials used in the API market in the Middle East and North Africa are mainly chemicals, organic compounds and biotech inputs purchased worldwide for pharmaceutical manufacturing. Arab Active Pharmaceutical Ingredient (API) Market is consistently competitive in terms of pricing due to lower cost of production as well as government push for local manufacturing. Prices can differ significantly depending on conditions of the market and specific API, but its main objective is to be competitive with worldwide providers while using regional benefits such as closeness and knowledge of rules.
• Lucintel forecasts that synthetic chemical will remain the largest segment over the forecast period due to continuous research and development activities and growing demand of synthetic chemical APIs in therapeutic areas.
• Within this market, cardiology will remain the largest therapeutic area segment over the forecast period due to increasing cardiovascular diseases and increase in geriatric population.
Country wise Outlook for the Middle Eastern and North African Active Pharmaceutical Ingredient (API) Market
The Middle Eastern and North African active pharmaceutical ingredient (API) market is experiencing significant global growth, driven expanding healthcare infrastructure, increasing pharmaceutical research and development activities, and strategic government initiatives promoting local manufacturing capabilities. This growth is further fueled by rising demand for affordable medications, regional economic development, and advancements in pharmaceutical technologies within the MENA region.
Emerging Trends in the Middle Eastern and North African Active Pharmaceutical Ingredient (API) Market
Emerging trends in the Middle Eastern and North African active pharmaceutical ingredient (API) market shaping its future applications and market dynamics:
• Growing Emphasis on Developing Local Manufacturing and Self-Sufficiency: The need to develop local capacities for manufacturing active pharmaceutical ingredients (APIs) within MENA countries has increased. Governments and pharmaceutical companies are investing in infrastructure development, technology transfer, as well as regulatory frameworks so as to reduce reliance on imports and improve self-sufficiency with regards API production.
• Increasingly Focusing on Biopharmaceuticals and Biotechnology: The MENA region is increasingly directed at biopharmaceuticals including monoclonal antibodies from biotech derived APIs, recombinant proteins, biosimilars etc. It is the reason for increasing growth of this segment due to investment into infrastructure in biotechnology and partnerships with global biopharma companies.
• Demand For Generic Drugs and API Outsourcing: Due to rising healthcare costs associated with the treatment of chronic diseases, there is an increasing demand for generic drugs in MENA countries. This has resulted in a shift of API production from high cost locations to cheaper ones situated within the region such as Egypt, Jordan or Saudi Arabia.
• Regulatory Harmonization and Compliance: Efforts have been made toward harmonizing regulations across all MENA countries thereby facilitating faster approval of APIs and finished pharmaceutical products. The alignment process with international guidelines like ICH (International Council for Harmonisation) standards improves market access conditions hence fostering competitiveness.
• Growing Focus on Sustainability and Green Chemistry: Sustainability practices together with green chemistry principles are now becoming popular trends within the field of API manufacturing around the world. Forging ahead, important steps are being taken by manufacturers’ regulators in MENA towards establishing environmentally friendly processes that minimize waste generation, use resources optimally etc.
A more than 150 page report to help in your business decisions. A sample figure with insights is shown below.
Recent Developments in the Middle Eastern and North African Active Pharmaceutical Ingredient (API) Market
Recent developments in Middle Eastern and North African active pharmaceutical ingredient (API) market which highlights ongoing innovations and advancements across different sectors:
1. Investment in Infrastructure: Countries such as Saudi Arabia and the UAE are investing in pharmaceutical infrastructure to improve domestic manufacturing capabilities and reduce reliance on imports.
2. Regulatory Improvements: Many countries are improving regulatory frameworks to expedite approval processes for APIs, encourage local production, and ease market entry.
3. Emphasis on Biopharmaceuticals: The emphasis is shifting towards biopharmaceuticals including biosimilars with initiatives that will nurture R&D in biotechnology and bioprocessing.
4. Alliances and partnerships: More collaborations between local manufacturers, international pharmaceutical companies, and research institutions to foster innovative ideas and technology transfer in API manufacturing.
5. Market Growth: Increase of drug markets across MENA region due to increased health care spending, population growth, and demand for cheaper drugs.
6. Covid-19 Effect: The pandemic has brought into focus the significance of building up local manufacturing capacities which has spurred quickening efforts towards API production along with securing supply chains within the region.
Strategic Growth Opportunities for Middle Eastern and North African Active Pharmaceutical Ingredient (API) Market
The Middle Eastern and North African Active Pharmaceutical Ingredient (API) market is very dynamic due to expanding healthcare infrastructure, increasing prevalence of chronic diseases, and evolving regulatory landscapes across the region. Some key strategic growth opportunities for this market include:
Explosive Pharmaceutical Consumption and Demand:
• Pharmaceutical consumption in the MENA region has been experiencing a surge due to population growth, increased life expectancy, and growing rates of chronic diseases such diabetes, cardiovascular disorders, and cancer. This demographic transition also results in an extensive usage of APIs that are necessary for production of drugs targeted at these ailments.
Local Manufacturing and Supply Chain Resilience:
• To ensure availability of medicines through local manufacturing and maintain uninterrupted supply chains, governments in Middle East and North Africa (MENA) region are increasingly encouraging manufacture of pharmaceuticals. Therefore this push includes attracting investments for API production facilities; encouraging technology transfer; and forging alliances with global pharma companies. As such, firms establishing local API-manufacturing units will leverage on this trend gaining government support as well as incentives.
Regulatory Reforms and Harmonization:
• Attempts to harmonize pharmaceutical standards and improve the quality of medicine by way of adjusted regulations have created better conditions for API manufacturers operating in the MENA region. Consequently efforts made include simplifying registration procedures; adherence to international quality standards (such as ICH guidelines); addressing counterfeit medications thus improving market transparency thereby increasing investor’s confidence. This means that only those producers who will meet regulatory requirements while maintaining high-quality levels would be able to expand themselves within the market.
Increasing Investments in Healthcare Infrastructure:
• Governments and private sector entities across the MENA region are pouring vast sums into healthcare infrastructure development such as hospitals, clinics or healthcare centers. All these necessitate a wide variety of drug supplies including APIs both for generic and branded types. API manufacturers who can align their product offerings with healthcare priorities within the area along key infrastructure plans could take advantage of this.
Rising Focus on Biopharmaceuticals and Specialty APIs:
• Interest in biopharmaceuticals and specialty APIs is on the rise in MENA due to advances in biotechnology personalize medicine, targeted therapies among other issues. In turn niche therapeutic areas for biologics, biosimilars and other high value-APIs have emerged as lucrative areas in which API manufacturers can invest in specialized production capabilities and research collaborations.
Strategic Partnerships and Collaborations:
• In order to enter into the MENA API market and expand its scale, collaboration with local pharmaceutical companies as well as research institutions and healthcare providers is crucial. This has led to various partnerships that facilitate the transfer of technology, knowledge sharing, access to regional distribution networks among others. Additionally joint ventures and licensing agreements help API manufactures navigate through local market dynamics thereby making it easier for them capitalize on growth opportunities.
Focus on Cost-Effective and Sustainable Manufacturing Practices:
• For API manufacturers operating in the Middle East and North Africa (MENA) region, this means that cost-effective manufacturing processes coupled with sustainable practices are increasingly becoming important. It is possible for companies that adopt efficient production technologies, optimize resource utilization while embracing green manufacturing initiatives to attain competitive advantages all these without contravening ever tightening environmental regulations as well as sustainability expectations.
By taking advantage of these strategic growth opportunities, the Middle Eastern and North African active pharmaceutical ingredient (API) market can realize its full potential and transform numerous industries through enhanced production capabilities, regulatory alignment, and increased integration into global pharmaceutical supply chains.
Middle Eastern and North African Active Pharmaceutical Ingredient (API) Market Driver and Challenges
Middle Eastern and North African active pharmaceutical ingredient (API) market plays a pivotal role across industries such as healthcare, pharmaceutical manufacturing, biotechnology, and research and development. Additionally, it supports regional economic growth and contributes to job creation and technological advancement within the region.
The key drivers for the Middle Eastern and North African active pharmaceutical ingredient (API) market include:
1. Government Support and Investment: The API market is driven by government support and investment aimed at building up local pharmaceutical manufacturing capabilities. These include incentives, subsidies and regulatory reforms for freer entry into the market.
2. Increasing Healthcare Expenditure: Throughout MENA area rising healthcare expenses are responsible for the need of more pharmaceutical products such as APIs to improve accessibility to medicines that will meet health care needs.
3. Growing Population and Chronic Diseases: The increase in population in this area coupled with an increase in chronic diseases drives demand for drugs therefore contributing to API production growth in fulfillment of therapeutic needs.
4. Strategic Geographical Position: This regionÄX%$%Xs strategic geographical location enhances trade routes, provides access to international markets, hence enriching opportunities for API exports and regional economic integration.
The challenges facing the Middle Eastern and North African active pharmaceutical ingredient (API) market include:
1. Dependency on Imports: The Middle East has traditionally been heavily reliant on imported APIs which has led to supply chain risks, fluctuating prices and potential shortages during global disruptions.
2. Quality and Regulatory Compliance: For local manufacturers who want to be globally competitive or who would like to meet export specifications, it is a challenge trying to ensure that they comply with international quality standards and regulatory requirements.
3. Technological and Infrastructure Gaps: Pharmaceutical manufacturing in this region faces limitations due to inadequate technological knowhow as well as infrastructure gaps consequently making it impossible to scale API production upwards or adopt advanced manufacturing processes
4. Market Fragmentation: This leads to complex market access rules since there are numerous jurisdictions having different regulatory setups as well healthcare infrastructures thereby making it difficult to harmonize standards within the region.
Innovations in material science and manufacturing processes have led to a strong demand for active pharmaceutical ingredients (APIs) in the Middle East and North Africa, driven by advancements like sustainable production methods and enhanced purity standards. These developments are supporting regional pharmaceutical industries in achieving greater self-sufficiency and meeting global quality requirements.
Middle Eastern and North African Active Pharmaceutical Ingredient (API) Suppliers and their Market Shares
In this globally competitive market, several key players such as Pfizer, Teva Pharmaceutical, GSK, Sun Pharmaceutical, Novartis, etc. dominate the market and contribute to industry’s growth and innovation. These players capture maximum market share. To know the current market share of each of major players contact us.
Companies in the market compete on the basis of product quality offered. Major players in this market focus on expanding their manufacturing facilities, R&D investments, infrastructural development, and leverage integration opportunities across the value chain. With these strategies, active pharmaceutical ingredient companies cater to increasing demand, ensure competitive effectiveness, develop innovative products & technologies, reduce production costs, and expand their customer base. Some of the active pharmaceutical ingredient companies profiled in this report include-
• Pfizer
• Teva Pharmaceutical
• GSK
• Sun Pharmaceutical
• Novartis
• Aurobindo
• Merck& Co.
• BASF
• Julphar
• AbbVie
These companies have established themselves as leaders in the Middle Eastern and North African active pharmaceutical ingredient (API) market, with extensive product portfolios, global presence, and strong research and development capabilities. They continually strive to enhance their market positions through strategic partnerships, mergers and acquisitions, and product innovations.
The market share dynamics within the Middle Eastern and North African active pharmaceutical ingredient (API) market are evolving, with the entry of new players and the emergence of innovative Middle Eastern and North African Active Pharmaceutical Ingredient (API) technologies. Additionally, collaborations between material suppliers, manufacturers, and end-users are fostering technological advancements and expanding market opportunities.
Middle Eastern and North African Active Pharmaceutical Ingredient (API) Market by Segment
Major segments of the Middle Eastern and North African Active Pharmaceutical Ingredient (API) market that are experiencing significant growth include biopharmaceuticals, generics, and specialty pharmaceuticals. Biopharmaceutical APIs, such as biosimilars and biologics, are gaining traction due to advancements in biotechnology and increasing investments in healthcare infrastructure. The generics segment continues to expand rapidly, driven by patent expirations of branded drugs and the regionÄX%$%Xs focus on affordability and accessibility of medicines. Additionally, specialty pharmaceuticals, including APIs for chronic disease management and personalized therapies, are seeing growth with rising healthcare expenditures and an aging population. These segments reflect a shift towards innovative treatments and sustainable healthcare solutions across the MENA region.
This Middle Eastern and North African Active Pharmaceutical Ingredient (API) market report provides a comprehensive analysis of the marketÄX%$%Xs current trends, growth drivers, challenges, and future prospects in all major segments like above. It covers various segments, including Middle Eastern and North African Active Pharmaceutical Ingredient (API) types, synthesis type, manufacturer type, therapeutic area, product form, molecule type, and drug type. The report offers insights into regional dynamics, highlighting the major markets for Middle Eastern and North African Active Pharmaceutical Ingredient (API) market and their growth potentials. The study includes trends and forecast for the Middle Eastern and North African Active Pharmaceutical Ingredient (API) market by synthesis type, manufacturer type, therapeutic area, product form, molecule type, and drug type as follows:
Middle Eastern and North African Active Pharmaceutical Ingredient Market by Synthesis Type [Value ($ Million) for 2018 – 2030]:
• Synthetic Chemical
• Biotech and Biologics
• Plant Extracts
• Fermentation
• Others
Middle Eastern and North African Active Pharmaceutical Ingredient Market by Manufacturer Type [Value ($ Million) for 2018 – 2030]:
• Captive APIs
• Merchant APIs
Middle Eastern and North African Active Pharmaceutical Ingredient Market by Therapeutic Area [Value ($ Million) for 2018 – 2030]:
• Cardiology
• Oncology
• Neurology
• Ophthalmology
• Orthopedics
• Pulmonology
• Aliment
• Anti-Inflammatory
• Others
Middle Eastern and North African Active Pharmaceutical Ingredient Market by Product Form [Value ($ Million) for 2018 – 2030]:
• Liquid API
• Powder API
Middle Eastern and North African Active Pharmaceutical Ingredient Market by Molecule Type [Value ($ Million) for 2018 – 2030]:
• Large Molecule
• Small Molecule
Middle Eastern and North African Active Pharmaceutical Ingredient Market by Drug Type [Value ($ Million) for 2018 – 2030]:
• Branded
• Generic
• Over-the-Counter
Features of the Middle Eastern and North African Active Pharmaceutical Ingredient (API) Market
• Market Size Estimates: Middle Eastern and North African active pharmaceutical ingredient market size estimation in terms of value ($M).
• Trend and Forecast Analysis: Market trends (2018-2023) and forecast (2024-2030) by various segments.
• Segmentation Analysis: Middle Eastern and North African active pharmaceutical ingredient market size by various segments, such as synthesis type, manufacturer type, therapeutic area, product form, molecular type, and drug type in terms of value.
• Country Analysis: Middle Eastern and North African active pharmaceutical ingredient market breakdown by the Saudi Arabia.
• Growth Opportunities: Analysis of growth opportunities in different synthesis types, manufacturer types, therapeutic areas, product forms, molecular types, and drug types for the Middle Eastern and North African active pharmaceutical ingredient market.
• Strategic Analysis: This includes M&A, new product development, and competitive landscape of the Middle Eastern and North African active pharmaceutical ingredient market.
• Analysis of competitive intensity of the industry based on Porter’s Five Forces model
FAQ
Q1. What is the Middle Eastern and North African active pharmaceutical ingredient market size?
Answer: The global Middle Eastern and North African active pharmaceutical ingredient market is expected to reach an estimated $5 billion by 2030.
Q2. What is the growth forecast for Middle Eastern and North African active pharmaceutical ingredient market?
Answer: The Middle Eastern and North African active pharmaceutical ingredient market is expected to grow at a CAGR of 5% from 2023 to 2030.
Q3. What are the major drivers influencing the growth of the Middle Eastern and North African active pharmaceutical ingredient market?
Answer: The major drivers for this market are rise in growth of pharmaceutical production and research and development of new drugs.
Q4. What are the major therapeutic areas for the Middle Eastern and North African active pharmaceutical ingredient market?
Answer: Cardiology, oncology, neurology, ophthalmology, orthopedics, pulmonology, aliment, and anti-Inflammatory are the major therapeutic areas of the Middle Eastern and North African active pharmaceutical ingredient market.
Q5. What are the emerging trends in Middle Eastern and North African active pharmaceutical ingredient market?
Answer: Emerging trends, which have a direct impact on the dynamics of the industry, include growing emphasis on developing local manufacturing and self-sufficiency, increasingly focusing on biopharmaceuticals and biotechnology, demand for generic drugs and APIS outsourcing, regulatory harmonization and compliance, growing focus on sustainability and green chemistry
Q6. Who are the key Middle Eastern and North African active pharmaceutical ingredient companies?
Answer: Some of the key Middle Eastern and North African active pharmaceutical ingredient companies are as follows:
• Pfizer
• Teva Pharmaceutical
• GSK
• Sun Pharmaceutical
• Novartis
• Aurobindo
• Merck& Co.
• BASF
• Julphar
• AbbVie
Q7. Which will be the largest synthesis type segment in the Middle Eastern and North African active pharmaceutical ingredient market in the future?
Answer: Lucintel forecasts that synthetic chemical will remain the largest synthesis segment over the forecast period due to continuous research and development activities and growing demand for synthetic chemical APIs in therapeutic areas.
Q8. In Middle Eastern and North African active pharmaceutical ingredient market, which region is expected to be the largest in the next five years?
Answer: Asia Pacific is expected to be the largest region over the next five years.
Q9. Do we receive customization in this report?
Answer: Yes, Lucintel provides 10% customization without any additional cost.
This report answers following 11 key questions
Q.1 What are some of the most promising potential, high-growth opportunities for the Middle Eastern and North African active pharmaceutical ingredient market by synthesis type (synthetic chemical, biotech and biologics, plant extracts, fermentation, and others), manufacturer type (captive APIs and merchant APIs), therapeutic area (cardiology, oncology, neurology, ophthalmology, orthopedics, pulmonology, aliment, anti-inflammatory, and others ), product form (liquid API and powder API), molecule type (large molecule and small molecule), and drug type (branded, generic, and over-the-counter)?
Q.2 Which segments will grow at a faster pace and why?
Q.3 Which country will grow at a faster pace and why?
Q.4 What are the key factors affecting market dynamics? What are the drivers and challenges of the Middle Eastern and North African active pharmaceutical ingredient market?
Q.5 What are the business risks and threats to the Middle Eastern and North African active pharmaceutical ingredient market?
Q.6 What are the emerging trends in the active pharmaceutical ingredient market and the reasons behind them?
Q.7 What are some changing demands of customers in this active pharmaceutical ingredient market?
Q.8 What are the new developments in this active pharmaceutical ingredient market? Which companies are leading these developments?
Q.9 Who are the major players in this active pharmaceutical ingredient market? What strategic initiatives are being implemented by key players for business growth?
Q.10 What are some of the competitive products and processes in this active pharmaceutical ingredient market, and how big of a threat do they pose for loss of market share via material or product substitution?
Q.11 What M&A activities did take place in the last five years in the Middle Eastern and North African active pharmaceutical ingredient market?
For any questions related to Middle Eastern and North African active pharmaceutical ingredient (API) market or related to Middle Eastern and North African active pharmaceutical ingredient companies, Middle Eastern and North African active pharmaceutical ingredient market size, Middle Eastern and North African active pharmaceutical ingredient market share, Middle Eastern and North African active pharmaceutical ingredient market growth, Middle Eastern and North African active pharmaceutical ingredient market research, write Lucintel analyst at email: helpdesk@lucintel.com we will be glad to get back to you soon.