Interventional Cardiology and Peripheral Vascular Device Market Trends and Forecast
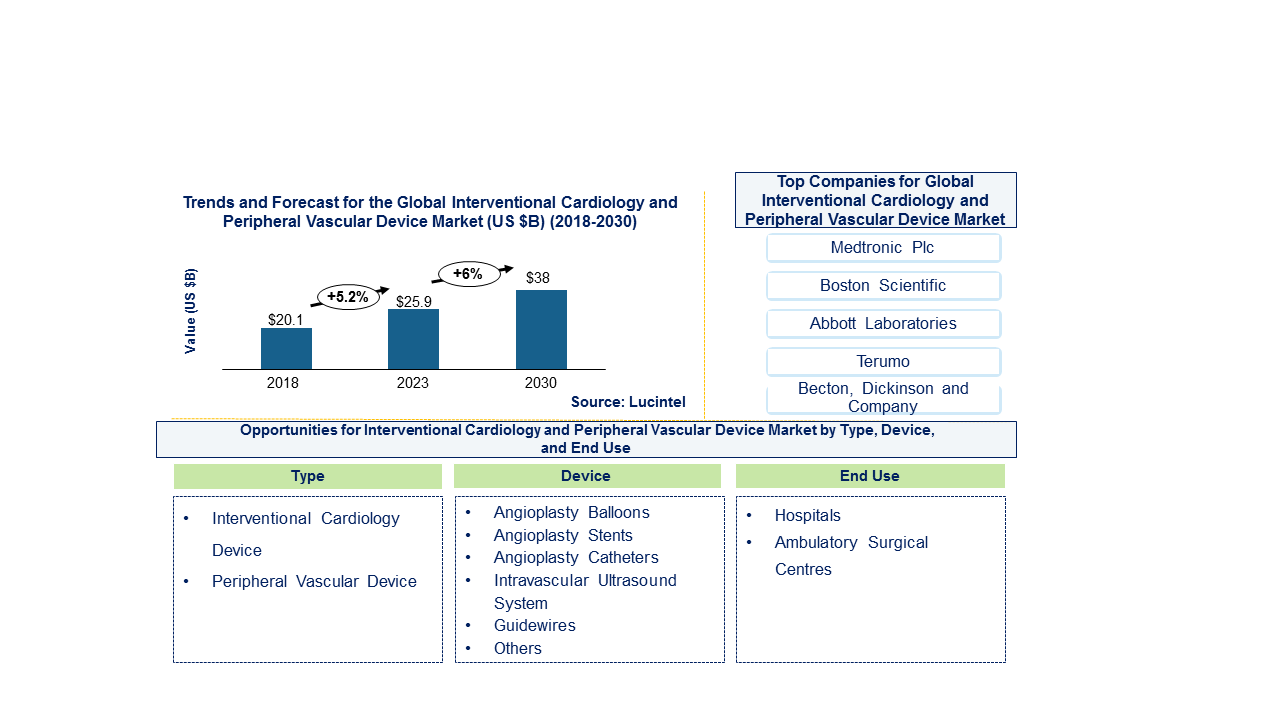
The future of the global interventional cardiology and peripheral vascular device market looks good with opportunities in hospitals and ambulatory surgical centers. The global interventional cardiology and peripheral vascular device market is expected to reach an estimated $38 billion by 2030, and it is forecast to grow at a CAGR of 6% from 2024 to 2030. The major drivers are growing elderly population, rise in prevalence of chronic diseases, and advancements in medical technology and research.
The raw materials used in the production of interventional cardiology and peripheral vascular devices vary based on the specific device and its application. Common materials include metals such as stainless steel, nitinol (a nickel-titanium alloy), and cobalt-chromium alloys, chosen for their biocompatibility, strength, and flexibility. Polymers like polyurethane and polyethylene are used for catheter components due to their flexibility and compatibility with body tissues. Bioresorbable materials, such as polylactic acid (PLA) and polyglycolic acid (PGA), are gaining prominence for devices that degrade over time within the body. These materials undergo rigorous testing and compliance with medical device regulations to ensure safety and efficacy in clinical use. The prices of interventional cardiology and peripheral vascular devices vary widely depending on factors such as device complexity, materials used, and technological advancements. Compared to traditional surgical interventions, interventional devices can be costlier upfront due to their advanced engineering and precise manufacturing processes. However, these devices often offer reduced hospitalization times and faster recovery, potentially lowering overall healthcare costs. Competition in the market drives pricing strategies, with established companies and new entrants vying to offer innovative solutions at competitive prices to healthcare providers globally. Market dynamics, including regulatory requirements and reimbursement policies, also influence pricing strategies in this highly specialized sector.
• Lucintel forecasts that interventional cardiology and peripheral vascular devices is expected to witness significant growth over the forecast period because of new technology innovations and aging population.
• Within global interventional cardiology and peripheral vascular device market, the largest segment by end user is projected to be hospitals due to the growing public-private partnerships to improve access to healthcare services along with rapid developments in healthcare infrastructure and improving access to healthcare services. These are the major driving factors for growth in this segment over the forecast period.
• APAC is expected to remain the largest region because it is a large pharmaceutical manufacturing base and it is witnessing increasing technological advancements in interventional cardiology and peripheral vascular devices.
• APAC is expected to witness the highest growth rate over the forecast period due to the presence of a large pool of respiratory patients, increasing healthcare expenditure, rapidly developing healthcare infrastructure, rising per capita income, growing middle-class population, and growing prevalence of tobacco smoking in this region.
Country wise Outlook for the Global Interventional Cardiology and Peripheral Vascular Device Market
Major players in the market are expanding their operations and forming strategic partnerships to strengthen their positions. Below image highlights recent developments by major interventional cardiology and peripheral vascular device producers in key regions: the USA, Germany, China, Japan, and India.
Emerging Trends in Interventional Cardiology and Peripheral Vascular Device Market
Emerging trends, which have a direct impact on the dynamics of the industry, include increasing demand for interventional cardiology and peripheral vascular device monitoring systems and the growing acceptance of leak detection systems in interventional cardiology and peripheral vascular device.
1. Emerging trends in the interventional cardiology and peripheral vascular device market include:
2. Technological Advancements: Innovations in device design and materials are leading to the development of next-generation stents, catheters, and other vascular devices. Drug-eluting stents, bioresorbable stents, and stent-grafts are becoming more advanced, offering better patient outcomes.
3. Minimally Invasive Procedures: There is a strong shift towards minimally invasive procedures, which reduce patient recovery times and hospital stays. Techniques like percutaneous coronary intervention (PCI) and endovascular aneurysm repair (EVAR) are becoming more refined and widely adopted.
4. Growing Use of Imaging Technologies: Advanced imaging technologies such as intravascular ultrasound (IVUS), optical coherence tomography (OCT), and fractional flow reserve (FFR) are enhancing the precision and efficacy of interventional procedures. These tools help in better visualization and assessment of vascular conditions.
5. Increased Prevalence of Cardiovascular Diseases: The rising incidence of cardiovascular diseases, driven by factors like aging populations, sedentary lifestyles, and unhealthy diets, is propelling the demand for interventional cardiology and peripheral vascular devices.
6. Development of Drug-Coated Balloons: Drug-coated balloons (DCBs) are emerging as a significant trend for treating peripheral artery disease (PAD). These devices deliver anti-proliferative drugs directly to the arterial wall, reducing the risk of restenosis.
7. Expansion of Structural Heart Interventions: Structural heart interventions, including transcatheter aortic valve replacement (TAVR) and mitral valve repair/replacement (TMVR), are expanding. These procedures provide minimally invasive options for patients with valve diseases.
A total of 104 figures / charts and 68 tables are provided in this 191-page report to help in your business decisions. A sample figure with some insights is shown below. To learn the scope of, benefits, companies researched and other details of interventional cardiology and peripheral vascular device market report, please download the report brochure.
Recent Developments by the Interventional Cardiology and Peripheral Vascular Device Market
Recent developments in the interventional cardiology and peripheral vascular device market reflect advancements in technology, strategic business moves, and increasing focus on patient outcomes. Here are some key updates:
• Next-Generation DES: Development of newer drug-eluting stents with better biocompatibility and longer-lasting drug release profiles to reduce restenosis and improve long-term outcomes.
• Bioresorbable Scaffolds: Continued innovation in bioresorbable vascular scaffolds that dissolve after delivering their therapeutic effect, leaving no permanent implant behind.
• Expanded Indications: Regulatory approvals for TAVR devices to be used in a broader range of patients, including those at low surgical risk.
• Improved Delivery Systems: Enhancements in delivery systems for TAVR devices to facilitate easier navigation and deployment, reducing procedural complications.
• Industry Consolidation: Continued consolidation within the market as major players acquire smaller companies to expand their product portfolios and technological capabilities. For example, Abbott’s acquisition of Walk Vascular to enhance its peripheral vascular offerings.
• Vertical Integration: Companies acquiring firms involved in different stages of the supply chain to streamline operations and reduce costs.
• Research Collaborations: Partnerships between medical device companies and research institutions to accelerate the development of new technologies and clinical solutions.
• Joint Ventures: Formation of joint ventures to leverage combined expertise and resources for the development and commercialization of innovative devices.
• Patient Comfort and Recovery: Development of devices and techniques that reduce procedural invasiveness, leading to quicker recovery times and improved patient comfort.
• Outpatient Procedures: Increasing capability to perform interventional cardiology and peripheral vascular procedures in outpatient settings, reducing healthcare costs and improving patient convenience.
• Personalized Medicine: Advances in personalized medicine approaches, where devices and treatments are tailored to the individual patient’s anatomical and physiological characteristics.
• Enhanced Patient Monitoring: Use of wearable devices and remote monitoring technologies to track patient health post-procedure and adjust treatments as necessary.
• FDA Approvals: Recent FDA approvals for new devices and expanded indications for existing devices, facilitating broader clinical use.
• EU MDR Compliance: Companies working to comply with the European Union’s Medical Device Regulation (MDR) to continue marketing their products in the European market.
• Increased Demand: Rising prevalence of cardiovascular diseases and PAD globally is driving demand for interventional cardiology and peripheral vascular devices.
• Technological Adoption: Higher adoption rates of advanced technologies by healthcare providers due to proven clinical benefits and cost-effectiveness.
These developments highlight the dynamic nature of the interventional cardiology and peripheral vascular device market, with ongoing innovations aimed at improving patient outcomes, procedural efficiency, and market expansion.
Strategic Growth Opportunities for Interventional Cardiology and Peripheral Vascular Device Market
Strategic growth opportunities in the interventional cardiology and peripheral vascular device market are driven by technological advancements, increasing prevalence of cardiovascular diseases, and evolving healthcare demands. Here are some key areas of opportunity:
Technological Innovations
• Drug-Eluting Stents (DES): Continued development of next-generation DES with improved safety profiles and efficacy for reducing restenosis rates.
• Bioresorbable Vascular Scaffolds: Expansion of bioresorbable technologies that provide temporary support while promoting natural healing in the arteries.
• Robotic-Assisted Procedures: Adoption of robotic systems to enhance precision and reduce complications during interventional procedures.
Emerging Markets
• Asia-Pacific and Latin America: Increasing investment in healthcare infrastructure and rising demand for advanced medical devices in emerging markets present significant growth potential.
• Rising Middle Class: Growing middle-class populations in developing regions are driving demand for better healthcare services and advanced medical technologies.
Telemedicine and Remote Monitoring
• Integration of Telehealth: Expanding telehealth capabilities for pre- and post-procedure monitoring, allowing for better patient management and follow-up.
• Wearable Devices: Development of wearable monitoring devices that can provide real-time data on cardiovascular health, facilitating timely interventions.
Regulatory and Reimbursement Landscape
• Streamlined Approvals: Ongoing efforts by regulatory bodies to streamline the approval process for innovative devices can accelerate market entry.
• Reimbursement Policies: Advocacy for favorable reimbursement policies for interventional cardiology procedures and devices to encourage adoption among healthcare providers.
Patient-Centric Solutions
• Customized Treatments: Focus on developing personalized treatment options based on patient-specific anatomical and physiological factors.
• Patient Education and Engagement: Implementing educational programs and tools that empower patients to manage their cardiovascular health effectively.
Strategic Partnerships and Collaborations
• Collaboration with Tech Companies: Partnering with technology firms to integrate AI, machine learning, and data analytics into interventional devices and workflows.
• Academic Partnerships: Collaborating with academic institutions for research and clinical trials to innovate and validate new technologies.
Sustainability Initiatives
• Eco-Friendly Products: Development of sustainable and environmentally friendly devices that comply with increasing regulations on waste and environmental impact.
• Recycling Programs: Implementing recycling programs for single-use devices to reduce the environmental footprint.
Expanded Applications
• Peripheral Vascular Interventions: Growth in peripheral vascular interventions, including treatments for peripheral artery disease (PAD), creating opportunities for new device development.
• Heart Failure Management: Innovations in devices targeting heart failure management, such as left ventricular assist devices (LVADs) and implantable devices.
The interventional cardiology and peripheral vascular device market offers significant growth opportunities driven by technological innovation, demographic trends, and evolving healthcare practices. Companies that focus on these areas can enhance their competitive positioning and drive market expansion.
Interventional Cardiology and Peripheral Vascular Device Market Driver and Challenges
Drivers in the Interventional Cardiology and Peripheral Vascular Device Market
1. Rising Prevalence of Cardiovascular Diseases
Increasing incidence of heart diseases, including coronary artery disease and peripheral artery disease, drives demand for interventional devices.
2. Aging Population
A growing elderly population is more susceptible to cardiovascular issues, leading to higher utilization of interventional cardiology procedures.
3. Technological Advancements
Innovations in device technology, such as drug-eluting stents, bioresorbable scaffolds, and robotic-assisted systems, improve patient outcomes and drive adoption.
4. Increasing Awareness and Screening
Greater awareness of cardiovascular health and advancements in screening methods encourage early detection and treatment, increasing demand for interventional procedures.
5. Minimally Invasive Procedures
A shift toward minimally invasive techniques reduces recovery times and complications, making interventional cardiology more appealing to patients and providers.
6. Investment in Healthcare Infrastructure
Increased investment in healthcare facilities and technologies enhances access to interventional procedures and devices, especially in emerging markets.
Challenges in the Interventional Cardiology and Peripheral Vascular Device Market
1. Regulatory Hurdles
Navigating complex regulatory approval processes can delay product launches and increase costs for manufacturers.
2. High Costs of Devices and Procedures
The high costs associated with advanced interventional devices can limit access for patients, especially in developing regions with constrained healthcare budgets.
3. Competition and Market Saturation
Intense competition among established players and new entrants can lead to market saturation, putting pressure on pricing and profit margins.
4. Technological Complexity
The increasing complexity of devices requires specialized training for healthcare professionals, which can pose challenges in adoption and utilization.
5. Economic Factors
Economic downturns and changes in reimbursement policies can affect hospital budgets and the adoption of new technologies in interventional cardiology.
6. Patient Risk and Safety Concerns
Concerns regarding the risks associated with interventional procedures, including complications and adverse events, can affect patient willingness to undergo treatment.
The interventional cardiology and peripheral vascular device market is driven by a combination of demographic trends, technological advancements, and a growing focus on minimally invasive treatments. However, challenges such as regulatory complexities, high costs, and competition must be addressed to ensure continued growth and accessibility of these essential medical technologies.
Interventional Cardiology and Peripheral Vascular Device Suppliers and their Market Shares
In this globally competitive market, several key players such as Abbott Laboratories, Boston Scientific Corporation, Edwards Lifesciences Corporation, Medtronics Public Limited Company, C. R. Bard, Inc., etc. dominate the market and contribute to industry’s growth and innovation. These players capture maximum market share. To know the current market share of each of major players contact us.
Companies in the market compete on the basis of product quality offered. Major players in this market focus on expanding their manufacturing facilities, R&D investments, infrastructural development, and leverage integration opportunities across the value chain. With these strategies, interventional cardiology and peripheral vascular device companies cater increasing demand, ensure competitive effectiveness, develop innovative products & technologies, reduce production costs, and expand their customer base. Some of the interventional cardiology and peripheral vascular device companies profiled in this report include:
• Abbott Laboratories
• Boston Scientific Corporation
• Edwards Lifesciences Corporation
• Medtronics Public Limited Company
• C. R. Bard, Inc.
• Cook Medical Inc.
• Terumo Corporation
• Cardinal Health Inc.
These companies have established themselves as leaders in the interventional cardiology and peripheral vascular device industry, with extensive product portfolios, global presence, and strong research and development capabilities. They continually strive to enhance their market positions through strategic partnerships, mergers and acquisitions, and product innovations.
The market share dynamics within the interventional cardiology and peripheral vascular device are evolving, with the entry of new players and the emergence of innovative interventional cardiology and peripheral vascular device technologies. Additionally, collaborations between material suppliers, manufacturers, and end-users are fostering technological advancements and expanding market opportunities.
Interventional Cardiology and Peripheral Vascular Device Market by Segments
In the interventional cardiology and peripheral vascular device market, major segments experiencing notable growth, driven by their effectiveness in reducing restenosis rates in coronary artery disease. The use of bioresorbable stents is also expanding, offering temporary scaffolding with the advantage of being absorbed by the body over time. Additionally, advancements in catheter technologies, including balloon catheters and atherectomy devices, are seeing increased adoption for both coronary and peripheral interventions due to their precision and minimally invasive nature. The structural heart devices segment, including transcatheter aortic valve replacement (TAVR) and mitral valve repair devices, is growing rapidly, addressing the needs of an aging population with valvular heart diseases. These segments are fueled by ongoing innovations, an aging global population, and rising incidences of cardiovascular diseases.
This interventional cardiology and peripheral vascular device market report provides a comprehensive analysis of the market's current trends, growth drivers, challenges, and future prospects in all major segments like above. It covers various segments, including type, device, and end use. The report offers insights into regional dynamics, highlighting the major markets for interventional cardiology and peripheral vascular device and their growth potentials. The study includes trends and forecast for the global interventional cardiology and peripheral vascular device market by type, device, end use and region as follows:
Interventional Cardiology and Peripheral Vascular Device Market by Type [Value ($ Million) from 2018 to 2030]:
• Interventional Cardiology Devices
• Peripheral Vascular Devices
Interventional Cardiology and Peripheral Vascular Device Market by Device [Value ($ Million) from 2018 to 2030]:
• Angioplasty Balloons
• Angioplasty Stents
• Angioplasty Catheters
• EVAR Stent Graft
• IVC Filter
• Embolic Protection Devices
• Guidewires
• Arteriotomy Closure Devices
• Intravascular Ultrasound System
• Synthetic Surgical Graft
Interventional Cardiology and Peripheral Vascular Device Market by End User [Value ($ Million) from 2018 and 2030]:
• Hospitals
• Ambulatory Surgical Centers
Interventional Cardiology and Peripheral Vascular Device Market by Region [Value ($ Million) from 2018 to 2030]:
• North America
o US
o Mexico
o Canada
• Europe
o United Kingdom
o Germany
o France
o Spain
• APAC
o China
o Japan
o India
• ROW
o Brazil
Features of Global Interventional Cardiology and Peripheral Vascular Device Market
• Market Size Estimates: Global interventional cardiology and peripheral vascular device market size estimation in terms of value ($M) shipment.
• Trend and Forecast Analysis: Market trends (2018-2023) and forecast (2024-2030) by various segments and regions.
• Segmentation Analysis: Global interventional cardiology and peripheral vascular device market size by various segments, such as type, end user, and regions in terms of value.
• Regional Analysis: Global interventional cardiology and peripheral vascular device market breakdown by North America, Europe, Asia Pacific, and the Rest of the World.
• Growth Opportunities: Analysis on growth opportunities in different type, end use, and regions for global interventional cardiology and peripheral vascular device market.
• Strategic Analysis: This includes M&A, new product development, and competitive landscape for the global interventional cardiology and peripheral vascular device market.
• Analysis of competitive intensity of the industry based on Porter’s Five Forces model.
If you are looking to expand your business in interventional cardiology and peripheral vascular device or adjacent markets, then contact us. We have done hundreds of strategic consulting projects in market entry, opportunity screening, due diligence, supply chain analysis, M & A, and more.
FAQ
Q1. What is the interventional cardiology and peripheral vascular device market size?
Answer: The global interventional cardiology and peripheral vascular device market is expected to reach an estimated $38 billion by 2030.
Q2. What is the growth forecast for the interventional cardiology and peripheral vascular device market?
Answer: The interventional cardiology and peripheral vascular device market is expected to grow at a CAGR of 6% from 2018 to 2030.
Q3. What are the major drivers influencing the growth of the interventional cardiology and peripheral vascular device market?
Answer: The major drivers of this market are growing elderly population, rise in prevalence of chronic diseases, and advancements in medical technology and research.
Q4. What are the major end use or end use industries for interventional cardiology and peripheral vascular devices?
Answer: Hospitals and ambulatory surgical centers are the major end users of interventional cardiology and peripheral vascular devices.
Q5. What are the emerging trends in the interventional cardiology and peripheral vascular device market?
Answer: Emerging trends, which have a direct impact on the dynamics of the industry, include a strong shift towards minimally invasive procedures, which reduce patient recovery times and hospital stays. Techniques like percutaneous coronary intervention (PCI) and endovascular aneurysm repair (EVAR) are becoming more refined and widely adopted.
Q6. Who are the key interventional cardiology and peripheral vascular device companies?
Answer: Some of the key interventional cardiology and peripheral vascular device companies are as follows:
• Abbott Laboratories
• Boston Scientific Corporation
• Edwards Lifesciences Corporation
• Medtronics Public Limited Company
• C. R. Bard, Inc.
• Cook Medical Inc.
• Terumo Corporation
• Cardinal Health Inc.
Q7.Which interventional cardiology and peripheral vascular device type segment will be the largest in the future?
Answer: Lucintel forecasts that interventional cardiology and peripheral vascular device is expected to witness significant growth over the forecast period because of new technology innovations and aging population.
Q8: In the interventional cardiology and peripheral vascular device market, which region is expected to be the largest in next 5 years?
Answer: APAC will remain the largest region and expected to witness the highest growth over next 5 years.
Q9. Do we receive customization in this report?
Answer: Yes, Lucintel provides 10% Customization Without any Additional Cost.
This report answers following 11 key questions
Q.1 What are some of the most promising, high-growth opportunities for the global interventional cardiology and peripheral vascular device market by type (interventional cardiology and peripheral vascular devices), device (angioplasty balloons, angioplasty stents, angioplasty catheters, EVAR stent graft, IVC filter, embolic protection devices, guidewires, arteriotomy closure devices, intravascular ultrasound system, and synthetic surgical graft), end user (hospitals and ambulatory surgical centers), and region (North America, Europe, Asia Pacific, and the Rest of the World)?
Q.2 Which segments will grow at a faster pace and why?
Q.3 Which regions will grow at a faster pace and why?
Q.4 What are the key factors affecting market dynamics? What are the drivers and challenges of the global interventional cardiology and peripheral vascular device market?
Q.5 What are the business risks and threats to the global interventional cardiology and peripheral vascular device market?
Q.6 What are emerging trends in this global interventional cardiology and peripheral vascular device market t and the reasons behind them?
Q.7 What are some changing demands of customers in the global interventional cardiology and peripheral vascular device market?
Q.8 What are the new developments in the global interventional cardiology and peripheral vascular device market? Which companies are leading these developments?
Q.9 Who are the major players in the global interventional cardiology and peripheral vascular device market? What strategic initiatives are being implemented by key players for business growth?
Q.10 What are some of the competitive products and processes in the global interventional cardiology and peripheral vascular device market, and how big of a threat do they pose for loss of market share via material or product substitution?
Q.11 What M&A activities did take place in the last five years in the global interventional cardiology and peripheral vascular device market?
For any questions related to interventional cardiology and peripheral vascular device market or related to interventional cardiology and peripheral vascular device market share, interventional cardiology and peripheral vascular device market analysis, and interventional cardiology and peripheral vascular device market size, write to Lucintel analysts at helpdesk@lucintel.com. We will be glad to get back to you soon.