3D Printing Medical Device Market Trends and Forecast
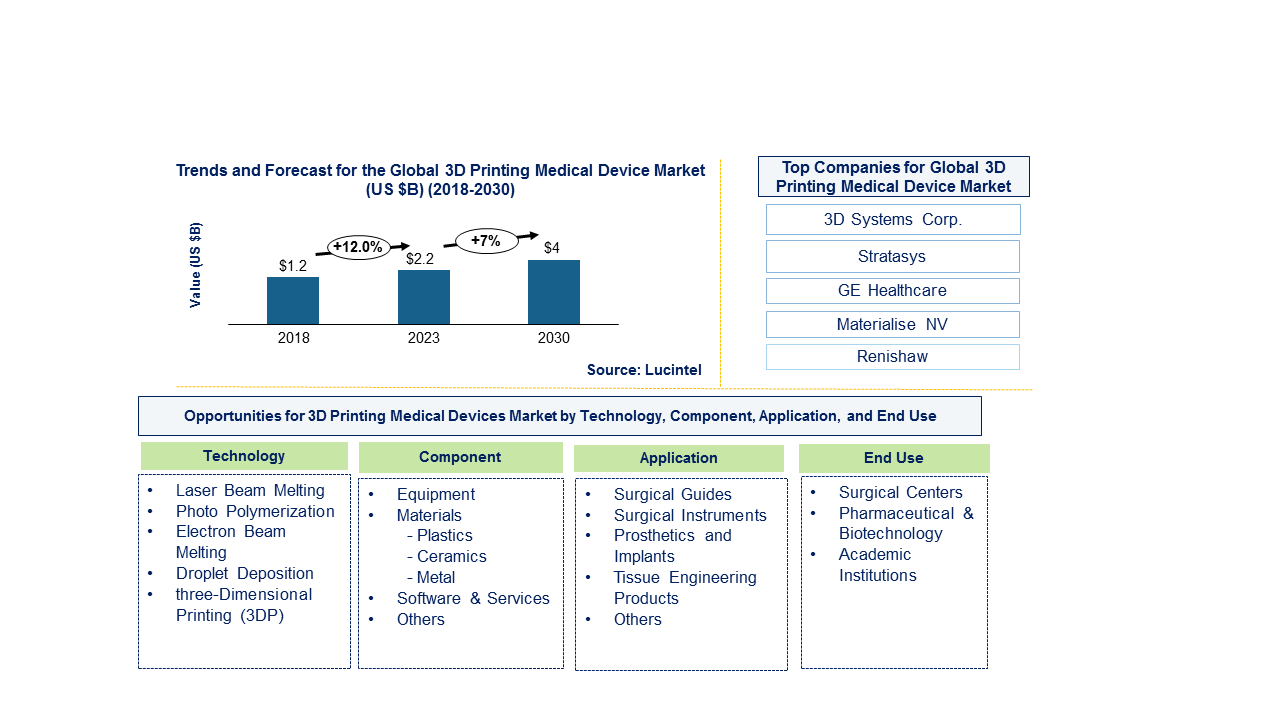
The future of the global 3D printing medical device market looks promising with opportunities in the medical & surgical centers, pharmaceutical & biotechnology, and academic institutions. The global 3D printing medical device market is expected to reach an estimated $4 billion by 2030 with a CAGR of 7% from 2023 to 2030. The major drivers for this market are technological advancements, rising prevalence of osteoarthritis, and increasing public-private funding for 3D printing activities
The raw materials used in 3D printing medical devices vary based on the specific application but commonly include biocompatible polymers (such as PLA, ABS, PEEK), metals (such as titanium, stainless steel), ceramics, and bio-inks for bio-printing. These materials are chosen for their ability to meet regulatory requirements, biocompatibility, and mechanical properties necessary for medical applications. The cost of 3D printing medical devices is influenced by factors like the complexity of design, choice of materials, manufacturing process (e.g., selective laser sintering, stereolithography), and regulatory compliance. While initial costs for 3D printing equipment and materials can be higher, the ability to customize designs and produce complex geometries often offsets traditional manufacturing costs in the long term. Competition in the market drives pricing strategies, with manufacturers aiming to offer competitive pricing while maintaining high standards of quality, reliability, and patient safety. Economic efficiencies gained through scale and technological advancements further contribute to pricing dynamics in the competitive landscape of 3D printing medical device.
• Lucintel forecasts that the photo polymerization technology based 3D printing market is expected to witness the highest growth over the forecast period due to the widespread application of this technology across the medical industry, such as manufacturing surgical guides (orthopedic and dental), prosthetics and implants, porous scaffolds, and dental restorations.
• Within the 3D printing technology market, equipment, materials, and software & services are major component type. The software & services market is expected to witness the highest growth due to the advent of ongoing technological advancements in software solutions to produce high-quality 3D printed medical products.
• North America is expected to remain the largest market during the forecast period mainly due to the rapid adoption of this technology, established medical infrastructure, and ongoing R&D activities. APAC is likely to witness the highest growth over the forecast period due to establishment of new 3D printing research, training, and education centers and growing efforts by leading market players for expanding their distribution networks in emerging Asian countries.
Country wise Outlook for the 3D Printing Medical Device Market
Emerging Trends in 3D Printing Medical Device Market
Emerging trends which have a direct impact on the dynamics of the 3D printing medical device industry include:
• Custom Implants and Prosthetics: Increasing adoption of patient-specific implants and prosthetics designed using 3D printing technology for better anatomical fit and improved patient outcomes.
• Advancements in 3D Bioprinting: Advancements in 3D bioprinting to create living tissues, organs, and scaffolds using bioinks and patient-derived cells for applications in regenerative medicine and tissue engineering.
• Surgical Instruments and Guides: Development of 3D printed surgical instruments, patient-specific surgical guides, and anatomical models for precise surgical planning, training, and execution.
• Dental Applications: Growing use of 3D printing in dentistry for manufacturing dental implants, crowns, bridges, and aligners with enhanced customization, accuracy, and patient comfort.
• Orthopedic Devices: Innovation in 3D printed orthopedic implants, such as spinal cages, hip and knee implants, and bone plates, offering improved biocompatibility and integration with natural bone tissue.
• FDA and Regulatory Advancements: Increasing regulatory approvals for 3D printed medical devices, ensuring safety, efficacy, and quality standards are met across various medical specialties.
• Smart Implants and Wearables: Integration of smart technologies, such as sensors and electronics, into 3D printed implants and wearable medical devices for real-time monitoring and enhanced patient care.
• Virtual Surgical Planning: Adoption of virtual reality (VR) and augmented reality (AR) technologies for preoperative planning and simulation using 3D printed models, improving surgical accuracy and reducing operating time.
• Advances in 3D Printing Materials: Advances in 3D printing materials including biocompatible polymers, ceramics, metals, and composite materials with enhanced mechanical properties, bioactivity, and sterilization capabilities.
A more than 150-page report to help in your business decisions. Sample figures with some insights are shown below.
Recent Developments in the 3D Printing Medical Device Market
Recent developments in 3D printing medical device market which highlights ongoing innovations and advancements across different sectors:
• Advancements in Patient-Specific Implants: There have been significant advancements in the production of patient-specific implants using 3D printing technology. Recent developments include the use of advanced imaging techniques such as CT scans and MRI to create digital models of patient anatomy, which are then used to design and fabricate customized implants tailored to individual patient specifications.
• Expansion of Point-of-Care Manufacturing: 3D printing technology is increasingly being used for point-of-care manufacturing of medical devices, allowing healthcare providers to produce customized implants and surgical tools on-site, reducing lead times and costs. Recent developments include the adoption of portable and desktop 3D printers in hospital settings for the rapid prototyping and production of patient-specific devices.
• Integration of Bioresorbable Materials: There's a growing interest in the use of bioresorbable materials for 3D printing medical devices, particularly implants that gradually dissolve or degrade in the body over time. Recent developments include the development of bioresorbable polymers and composite materials suitable for 3D printing applications, enabling the fabrication of temporary implants for orthopedic, cardiovascular, and soft tissue repair.
• Regulatory Advances and Standards Development: Regulatory agencies and standards organizations are making progress in establishing guidelines and standards for the use of 3D printing in the medical device industry. Recent developments include the publication of regulatory guidance documents and consensus standards addressing aspects such as material characterization, manufacturing process validation, and quality control requirements for 3D-printed medical devices.
• Innovation in Bio-printing and Tissue Engineering: 3D bio-printing technology is advancing rapidly, enabling the fabrication of living tissues and organ constructs for regenerative medicine and transplantation. Recent developments include the use of bio-ink formulations containing living cells, growth factors, and biomaterials to create complex tissue structures with vascularization and functionality, paving the way for personalized regenerative therapies.
Strategic Growth Opportunities for 3D Printing Medical Device Market
Some key strategic growth opportunities for this market include:
Customization and Personalization
• Patient-Specific Devices: Utilize 3D printing to create personalized medical implants, prosthetics, and surgical guides tailored to individual patient anatomy, improving treatment outcomes and patient satisfaction.
• Mass Customization: Offer scalable solutions for mass customization of medical devices, catering to diverse patient needs and enhancing product differentiation in the market.
Innovation in Complex Designs
• Complex Geometries: Leverage 3D printing capabilities to manufacture intricate and complex device designs that are challenging or impossible to achieve with traditional manufacturing methods, enhancing functionality and performance.
• Integration of Multiple Functions: Develop multi-functional medical devices, such as implants with drug delivery systems or sensors, using advanced 3D printing techniques to streamline treatment procedures and improve patient care.
Rapid Prototyping and Time-to-Market
• Accelerated Product Development: Utilize rapid prototyping capabilities of 3D printing to shorten design iterations and accelerate the development of new medical devices, reducing time-to-market and enhancing competitiveness.
• Iterative Design Optimization: Enable iterative design improvements based on real-time feedback from healthcare professionals and patients, ensuring product reliability, safety, and efficacy.
Supply Chain Resilience and Localized Manufacturing
• On-Demand Manufacturing: Implement decentralized manufacturing capabilities using 3D printing to enable on-demand production of medical devices, reducing inventory costs and responding quickly to regional healthcare needs.
• Reduced Dependency on Global Supply Chains: Mitigate risks associated with global supply chain disruptions by establishing local 3D printing facilities for medical device production, ensuring continuous supply and resilience.
Regulatory Compliance and Quality Assurance
• Standardization and Certification: Work closely with regulatory authorities to establish standards and obtain certifications for 3D printed medical devices, ensuring compliance with stringent healthcare regulations and quality standards.
• Quality Control Processes: Develop robust quality control processes and validation protocols specific to 3D printing technologies to guarantee the safety, efficacy, and consistency of medical devices throughout their lifecycle.
Collaboration and Partnerships
• Cross-Industry Collaboration: Form strategic partnerships with healthcare providers, research institutions, and technology firms to drive innovation, validate clinical applications, and expand market access for 3D printed medical devices.
• Joint Research Initiatives: Collaborate on research and development initiatives to explore new materials, printing technologies, and applications for 3D printed medical devices, fostering continuous innovation and market growth.
3D Printing Medical Device Market Driver and Challenges
Drivers:
The factors responsible for driving the 3D printing medical device market include:
1. Customization and Personalization: 3D printing allows for the creation of patient-specific medical devices tailored to individual anatomies, improving treatment outcomes and patient comfort.
2. Complex and Intricate Designs: Ability to produce complex geometries and structures that are difficult or impossible to achieve with traditional manufacturing methods, enhancing device functionality and performance.
3. Rapid Prototyping and Time-to-Market: Accelerated product development cycles through rapid prototyping, reducing time-to-market for new medical devices and facilitating iterative design improvements based on real-time feedback.
4. Innovation in Material Science: Advancements in materials suitable for 3D printing, including biocompatible polymers, metals, and ceramics, expanding the range of medical applications and improving device durability and biocompatibility.
5. Cost Efficiency and Resource Optimization: Cost-effective production of low-volume and custom devices without the need for expensive tooling or molds, enabling efficient resource allocation and reducing manufacturing waste.
Challenges Facing the 3D Printing Medical Device Market:
1. Regulatory Hurdles and Compliance: Stringent regulatory requirements for approval and certification of 3D printed medical devices, ensuring safety, efficacy, and quality standards compliance.
2. Quality Control and Standardization: Establishing consistent quality control processes and standards specific to 3D printing technologies to mitigate variability in device performance and ensure reproducibility.
3. Material Limitations and Biocompatibility: Limited availability of biocompatible materials suitable for medical device applications, coupled with challenges in achieving desired mechanical properties and long-term reliability.
4. Technological Limitations: Constraints in resolution, accuracy, and speed of current 3D printing technologies may impact the feasibility of producing intricate designs and meeting clinical requirements.
5. Cost of Implementation and Scaling: Initial investment costs associated with acquiring and maintaining 3D printing equipment, coupled with the need for specialized expertise in additive manufacturing and device design optimization.
6. Ethical and Legal Concerns: Addressing ethical considerations surrounding patient consent, data privacy, and intellectual property rights associated with 3D printed medical devices, particularly in personalized healthcare applications.
3D Printing Medical Device Suppliers and Their Market Shares
In this globally competitive market, several key players such as 3D Systems Corporation, Stratasys, GE Healthcare, Materialise NV, Renishaw, etc. dominate the market and contribute to industry’s growth and innovation. These players capture maximum market share. To know the current market share of each of major players contact us.
Companies in the market compete on the basis of product quality offered. Major players in this market focus on expanding their manufacturing facilities, R&D investments, infrastructural development, and leverage integration opportunities across the value chain. With these strategies 3D printing medical device companies cater increasing demand, ensure competitive effectiveness, develop innovative products & technologies, reduce production costs, and expand their customer base. Some of the 3D printing medical device companies profiled in this report include.
• 3D Systems Corporation
• Stratasys
• GE Healthcare
• Materialise NV
• Renishaw
These companies have established themselves as leaders in the 3D printing medical device industry, with extensive product portfolios, global presence, and strong research and development capabilities. They continually strive to enhance their market positions through strategic partnerships, mergers and acquisitions, and product innovations.
The market share dynamics within the 3D printing medical device are evolving, with the entry of new players and the emergence of innovative 3D printing medical device Additionally, collaborations between material suppliers, manufacturers, and end-users are fostering technological advancements and expanding market opportunities
3D Printing Medical Device Market by Segment
3D Printing Medical Device Market by Segment
Several key segments within the 3D printing medical device industry are experiencing notable growth. Segment is surgical instruments, as 3D printing enables the production of complex, precise instruments with intricate designs that enhance surgical precision and reduce surgical time. Additionally, the use of 3D printing for medical models and education is growing, facilitating better surgical planning, medical training, and patient education through the creation of accurate anatomical models. These segments collectively demonstrate the broadening impact of 3D printing technology in improving healthcare delivery and patient care.
This 3D printing medical device market report provides a comprehensive analysis of the market's current trends, growth drivers, challenges, and future prospects in all major segments. The report offers insights into regional dynamics, highlighting the major markets for 3D printing medical device and their growth potentials. The study includes trends and forecast for the 3D printing medical device market by technology, component, application, end use, and region as follows:
3D Printing Medical Device Market by Technology [Value ($ Million) from 2018 – 2030]:
• Laser Beam Melting
• Photo Polymerization
• Electron Beam Melting
• Droplet Deposition
• Three-Dimensional Printing (3DP)
3D Printing Medical Device Market by Component [Value ($ Million) from 2018 – 2030]:
• Equipment
• Materials
• Plastics
• Ceramic
• Metal
• Software & Services
• Others
3D Printing Medical Device Market by Application [Value ($ Million) from 2018 – 2030]:
• Surgical Guides
• Surgical Instruments
• Prosthetics and Implants
• Tissue Engineering Products
• Others
3D Printing Medical Device Market by End Use [Value ($ Million) from 2018 - 2030]:
• Surgical Centers
• Pharmaceutical & Biotechnology
• Academic Institutions
3D Printing Medical Device Market by Region [Value ($ Million) from 2018 – 2030]:
North America
• United States
• Canada
• Mexico
Europe
• United Kingdom
• Germany
• France
• Spain
• Italy
Asia Pacific
• China
• Japan
• India
The Rest of the World
• Brazil
Features of 3D Printing Medical Device Market
Market Size Estimates: 3D printing medical device market size estimation in terms of value ($M).
Trend and Forecast Analysis: Market trends (2018-2023) and forecast (2024-2030) by various segments and regions.
Segmentation Analysis: 3D printing medical device market size by various segments, such as technology, component, application, and end use.
Regional Analysis: 3D printing medical device market breakdown by North America, Europe, Asia Pacific, and the Rest of the World.
Growth Opportunities: Analysis on growth opportunities in different technologies, components, applications, end uses, and regions for 3D printing medical device market.
Strategic Analysis: This includes M&A and competitive landscape for the 3D printing medical device market.
Analysis of competitive intensity of the industry based on Porter’s Five Forces model.
If you are looking to expand your business in 3D printing medical device or adjacent markets, then contact us. We have done hundreds of strategic consulting projects in market entry, opportunity screening, due diligence, supply chain analysis, M & A, and more.
FAQ
Q1. What is the 3D printing medical device market size?
Answer: The global 3D printing medical device market is expected to reach an estimated $4 billion by 2030.
Q2. What is the growth forecast for 3D printing medical device market?
Answer: The 3D printing medical device market is expected to grow at a CAGR of 7% from 2023 to 2030.
Q3. What are the major drivers influencing the growth of the 3D printing medical device market?
Answer: The major drivers for this market are technological advancements, rising prevalence of osteoarthritis, and increasing public-private funding for 3D printing activities.
Q4. What are the major end uses for the 3D printing medical device market?
Answer: Surgical centers, pharmaceutical & biotechnology, and academic institutions are the major end uses of 3D printing medical devices.
Q5. What are the emerging trends in the 3D printing medical device market?
Answer: Emerging trends which have a direct impact on the dynamics of the 3D printing medical device industry include custom implants and prosthetics, advancements in 3d printing and smart implants and wearables.
Q6. Who are the key 3D printing medical device companies?
Answer: Some of the key 3D printing medical device companies are as follows:
• 3D Systems Corporation
• Stratasys
• GE Healthcare
• Materialise NV
• Renishaw
Q7.Which technology segment will witness the highest growth in the 3D printing medical device market in the Forecast period?
Answer: Lucintel forecasts that the photo polymerization technology based 3D printing market is expected to witness the highest growth over the forecast period due to widespread application of this technology across the medical industry, such as manufacturing surgical guides (orthopedic and dental), prosthetics and implants, porous scaffolds, and dental restorations.
Q8. In 3D printing medical device market, which region is expected to be the largest in the next five years?
Answer: North America is expected to be the largest region over the next five years.
Q9. Do we receive customization in this report?
Answer: Yes, Lucintel provides 10% customization without any additional cost.
This report answers following 11 key questions
Q.1 What are some of the most promising potential, high-growth opportunities for the 3D printing medical device market by technology (laser beam melting, photo polymerization, electron beam melting, droplet deposition, and three-dimensional printing (3DP)), component (equipment, materials (plastics, ceramics, and metal), software & services, and others), application (surgical guides, surgical instruments, prosthetics and implants, tissue engineering products, and others), end use (medical and surgical centers, pharmaceutical & biotechnology, and academic institutions), and region (North America, Europe, Asia Pacific, and the Rest of the World)?
Q.2 Which segments will grow at a faster pace and why?
Q.3 Which regions will grow at a faster pace and why?
Q.4 What are the key factors affecting market dynamics? What are the drivers and challenges of the 3D printing medical device market?
Q.5 What are the business risks and threats to the 3D printing medical device market?
Q.6 What are the emerging trends in this 3D printing medical device market and the reasons behind them?
Q.7 What are some changing demands of customers in the 3D printing medical device market?
Q.8 What are the new developments in the 3D printing medical device market? Which companies are leading these developments?
Q.9 Who are the major players in the 3D printing medical device market? What strategic initiatives are being implemented by key players for business growth?
Q.10 What are some of the competitive products and processes in the 3D printing medical device market, and how big of a threat do they pose for loss of market share via material or product substitution?
Q.11 What M&A activities did take place in the last five years in the 3D printing medical device market?
For any questions related to 3D printing medical device market or related to 3D printing medical device market analysis, 3D printing medical device market share, 3D printing medical device companies, top 3D printing medical device companies, 3D printing medical device manufacturers, and largest 3D printing medical device companies, write Lucintel analyst at email: helpdesk@lucintel.com we will be glad to get back to you soon.